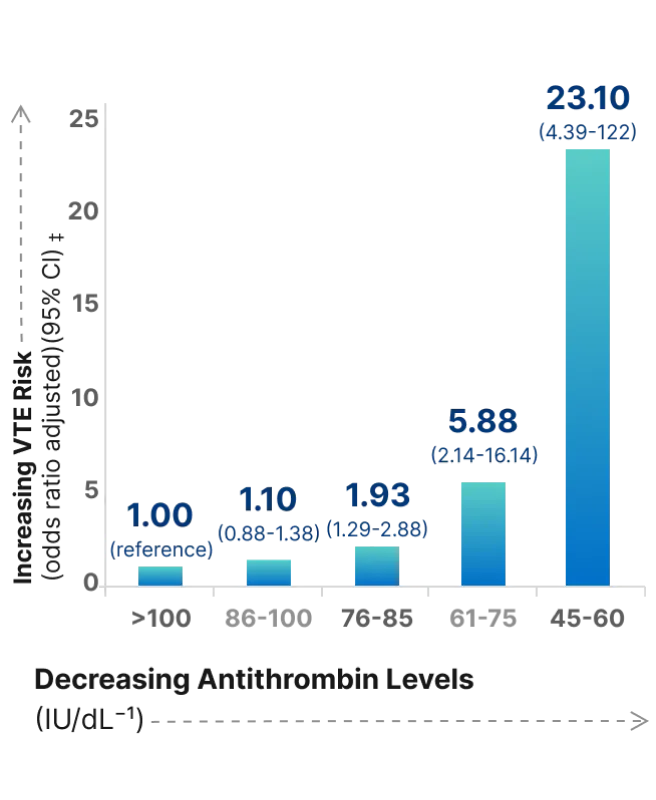
Even plasma levels that are considered normal (between 86 and 100 IU/dL) pose a significant risk for VTE21

The risk of VTE, when stratified for unprovoked VTE,* is 6 times higher for those with AT levels between 61% and 75%21
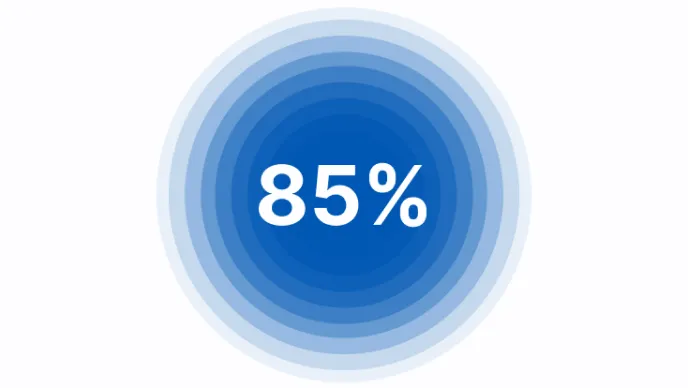
- 85% of patients with hereditary AT deficiency will have at least 1 thrombotic episode by age 507
- Nearly 70% of these patients will have an event before the age of 357
- Risk of unprovoked VTE* increases with decreasing AT levels