The anticoagulant effect of heparin is enhanced by concurrent treatment with THROMBATE III in patients with hereditary AT deficiency. Thus, in order to avoid bleeding, the dosage of heparin (or low molecular weight heparin) may need to be reduced during treatment with THROMBATE III.
THROMBATE III — Now approved in both pediatric and adult patients with hereditary antithrombin deficiency.
THROMBATE III is an effective choice for both pediatric and adult patients with hereditary antithrombin deficiency
Clinical studies have shown that THROMBATE III is an effective choice for both pediatric and adult patients with hATd and for the treatment and prevention of thromboembolism, including before, during, and after surgery and childbirth.1
THROMBATE III® (antithrombin III [human]) is indicated in both pediatric and adult patients with hereditary antithrombin deficiency for treatment and prevention of thromboembolism and for prevention of perioperative and peripartum thromboembolism.1

Why THROMBATE III?
In clinical studies, the most common adverse reactions (≥ 5% of subjects) were dizziness, chest discomfort, nausea, dysgeusia, and pain (cramps).

In clinical studies, THROMBATE III was proven effective for both pediatric and adult patients with hereditary antithrombin deficiency (hATd) for the treatment and prevention of thromboembolism, including before, during, and after surgery and childbirth.1
THROMBATE III is indicated in both pediatric and adult patients with hereditary antithrombin deficiency for treatment and prevention of thromboembolism and for prevention of perioperative and peripartum thromboembolism.1
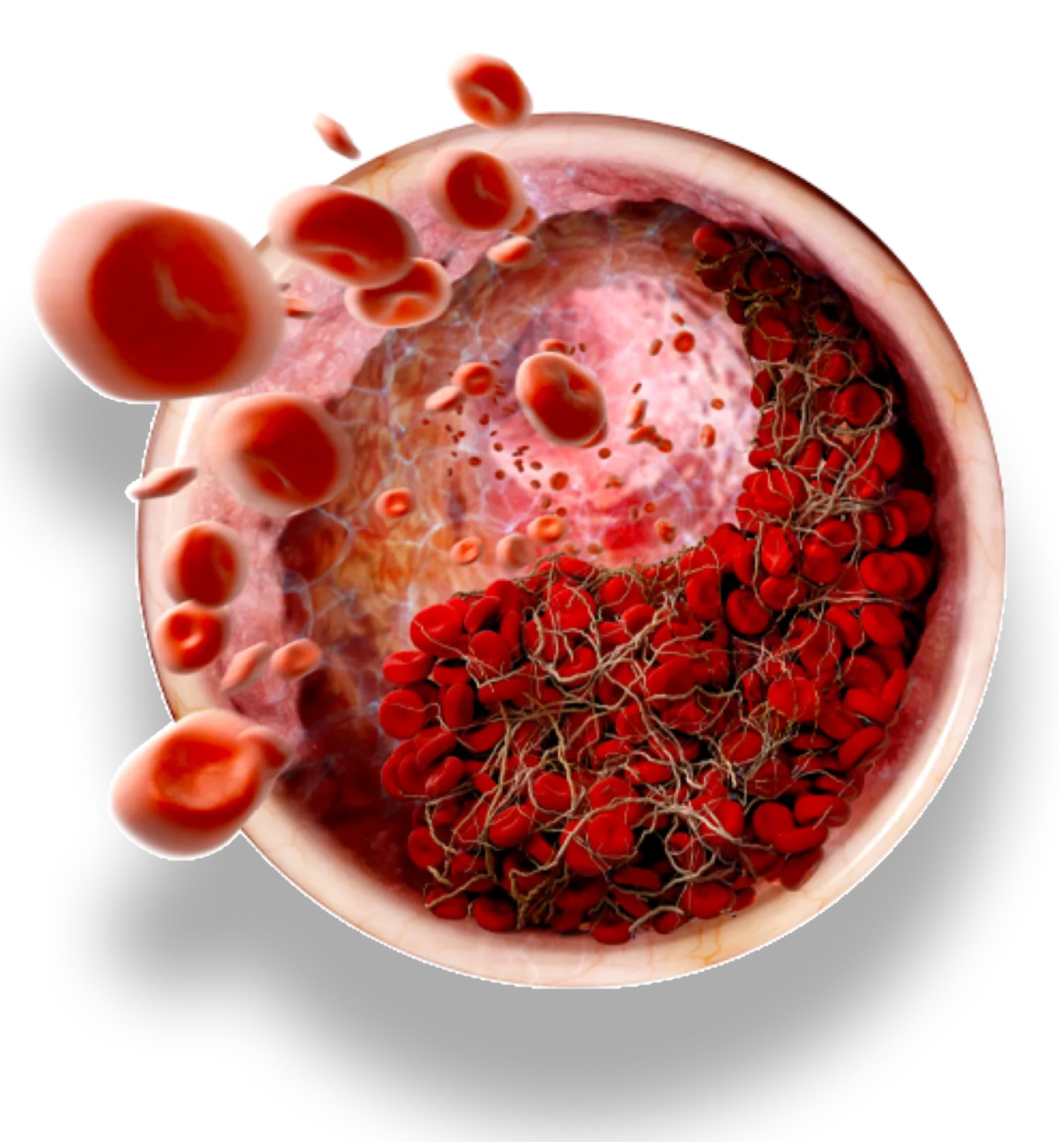
In both pediatric and adult patients with hATd, THROMBATE III is effective in preventing the expansion of a formed thrombus (clot) and formation of additional clots.1
No reports of thrombotic complications during obstetrical and surgical procedures¹
Childbirth/Surgery
- # of Procedures*
-
N = 13
- Outcome
-
No thrombosis or pulmonary embolism*
| Clinical Situation | # of Procedures* | Outcome |
|---|---|---|
|
Childbirth/Surgery |
N = 13 |
No thrombosis or pulmonary embolism* |
Patients diagnosed with hATd are at especially high risk for blood clots in certain situations such as: surgery, pregnancy, and childbirth, and when they already have a blood clot.2
Perform coagulation tests to avoid excessive or insufficient anticoagulation and monitor for bleeding or thrombosis. Measure functional plasma AT levels with amidolytic or clotting assays, do not use immunoassays.
THROMBATE III can provide predictable amounts of AT at the point of care¹
Has one dosing formula1
- Bolus intravenous infusion*
- Loading dose on day 1
Provides for convenient storage and reconstitution1
- No refrigeration required
- Store at room temperature (not to exceed 25ºC, 77ºF)
- No need to thaw
- Single-use vial (500 IU potency)
- Sterile water for injection (10 mL), transfer needle, and filter needle provided
Can be used before, during, and after surgery
Hypersensitivity reactions may occur. Should evidence of an acute hypersensitivity reaction be observed, promptly interrupt the infusion and begin appropriate treatment.
*Rate of administration adapted to the response of the individual patient, but administration of the entire dose in 10 to 20 minutes is generally well tolerated.
†Expressed as a percent of normal level based on functional AT assay. If laboratory testing is available, monitor plasma AT levels every 12 hours following the initial loading dose. THROMBATE III may be infused intravenously over 10 to 20 minutes.
Use THROMBATE III to replace what is normally present in the body1
The role of antithrombin in thrombosis
- 80% of the natural anticoagulant effect against thrombin is dependent on antithrombin2,3
- AT activity inhibits many clotting factors in the coagulation cascade2-6
Cofactor Relationship Between Antithrombin and Heparin
- Antithrombin inactivates multiple clotting factors in the coagulation cascade2,4
- Binds irreversibly/inhibits prothrombotic actions of factor Xa and thrombin
- The anticoagulant effects of heparin rely on its interaction with antithrombin
- Heparin depends on antithrombin as a cofactor7
- Heparin is ineffective in the absence or near absence of antithrombin4
- The anticoagulant activity of antithrombin is accelerated >1000X when bound to administered heparin4
- When antithrombin levels are deficient, activated procoagulant proteins circulate longer, increasing the risk of thrombosis8
- Signs of heparin (enoxaparin) resistance could suggest an inherited clotting disorder, such as hereditary AT deficiency2,3,9,10
Impact of Hereditary Antithrombin Deficiency on Heparin Action
- Patients with AT deficiency may have resistance to therapy with heparin and may require higher heparin doses for achievement of therapeutic activated partial thromboplastin time (aPTT) and protective anticoagulation, which may be the first clue of the underlying defect.2,9
- Increasing heparin doses without considering the possibility of hereditary antithrombin deficiency can have deleterious effects11
- Inadequate anticoagulation despite large intraoperative heparin doses
- Excessive heparin dosing can lead to increased postoperative bleeding
The anticoagulant effect of heparin is enhanced by concurrent treatment with THROMBATE III in patients with hereditary AT deficiency. Thus, in order to avoid bleeding, the dosage of heparin (or low molecular weight heparin) may need to be reduced during treatment with THROMBATE III.
Antithrombin deficiency is the most common cause of low heparin response7,9
Dr. Stephen Bader discusses important considerations for before, during, and after surgery
- Heparin resistance, or responsiveness issues, can occur in heparin-requiring surgeries, and administering additional heparin to overcome resistance could lead to bleeding at the end of the procedure
- Low heparin response is commonly defined as needing a daily dose in excess of 35,000 units/day
- Options to diagnose low heparin response in the operating room are limited, but interventions should be utilized to achieve the targeted ACT
- After the surgery, physicians may consider hematologic workup to make sure that a patient with low heparin response does not have hereditary antithrombin deficiency
- Patients with hereditary antithrombin deficiency need to be identified for appropriate management postoperatively and during future surgeries and life events, such as childbirth, which further increase their risk of thrombosis
Mitigating VTE Risk: Functional Evaluation of AT
- The risks of hereditary antithrombin deficiency can be mitigated with functional evaluation of antithrombin activity, including laboratory testing, as well as with long-term management to address and reduce risks during future procedures or other high-risk life events5
Whom to Test12
-
- Unexplained VTE at a younger age (<50 years)
- Recurrent spontaneous or unusually extensive spontaneous VTE
- Unexplained arterial thromboembolism in a younger patient
- Unexplained VTE at an unusual site
- Recurrence of VTE while adequatedly anticoagulated
- Family history of spontaneous VTE
- Family history of known thrombophilia, even if patient is asymptomatic
How to Test: Functional Antithrombin Assays
-
- Functional anththrombin assays are most commonly used and are recommended for initial testing for antithrombin deficiency7
- Initial testing should be completed a few weeks after thrombotic event—heparin use may decrease antithrombin levels by 30%12
- High specificity and sensitivity; positive predictive value = 96%7
Management12
-
- Asymptomatic individuals with antithrombin deficiency typically are not started on long-term anticoagulation; however they need to receive DVT prophylaxis in high-risk situations, such as surgery, pregnancy, or childbirth
- Patients with antithrombin deficiency who have had a VTE event should be considered for long-term anticoagulation
| Mitigating VTE Risk | |
|---|---|
|
Whom to Test12 |
|
|
How to Test: Functional Antithrombin Assays |
|
|
Management12 |
|
The effect of drugs that use antithrombin to exert their anticoagulation may be altered when THROMBATE III is added or withdrawn. Regularly perform coagulation tests suitable for the anticoagulant used (eg, aPTT and anti-Factor Xa activity) to avoid excessive or insufficient anticoagulation. Additionally, monitor the patients for the occurrence of bleeding or thrombosis.
THROMBATE III reduces VTE Risk
Dr. Bader talks about surgery in patients with hATd
THROMBATE III delivers 50x more antithrombin (AT) than the same amount of fresh frozen plasma (FFP)
- A vial of THROMBATE III contains concentrated AT factor, while a bag of FFP contains AT as well as varying amounts of other plasma components.1,13
Properties of antithrombin (AT) concentrate and fresh frozen plasma (FFP)
Indication
- THROMBATE III1
-
Indicated in both pediatric and adult patients with hereditary antithrombin deficiency (hATd) for treatment and prevention of thromboembolism and for prevention of perioperative and peripartum thromboembolism
- FFP13
-
Indicated in the management of patients with selected coagulation factor deficiencies, congenital or acquired, for which no specific coagulation concentrates are available
How supplied/volume
- THROMBATE III1
-
Single-dose 10-mL vial (500-IU potency)
- FFP13
-
Supplied in 200- to 250-mL bags (on average)
Concentration
- THROMBATE III1
-
50 IU/mL AT concentration (after reconstitution with 10 mL sterile water for injection)
- FFP13
-
~1 IU/mL AT concentration
Use
- THROMBATE III1
-
Intravenous bolus infusion, regardless of ABO status. Can be readily available at the point of care
- FFP13
-
Needs to be thawed prior to use. Intravenous infusion. Plasma must be ABO compatible with the recipient's red blood cells
Content
- THROMBATE III1
-
THROMBATE III provides predictable amounts of AT
- FFP13
-
Contains AT plus other plasma components in varying levels
Half-life
- THROMBATE III1
-
The half-life of THROMBATE III is similar to endogenous AT1,14
- FFP13
-
The components of FFP have varying half-lives
Storage
- THROMBATE III1
-
THROMBATE III can be stored at room temperature (up to 77°F) for up to 36 months. Do not freeze
- FFP13
-
FFP should be stored at −18°C (0°F) or colder. Infuse immediately after thawing or store at 1-6°C (34-43°F)
Process
- THROMBATE III1
-
THROMBATE III is produced from human plasma— it is fractionated and purified to yield concentrated antithrombin
- FFP13
-
Centrifuged, separated, and frozen solid at −18°C (0°F) within 8 hours of collection
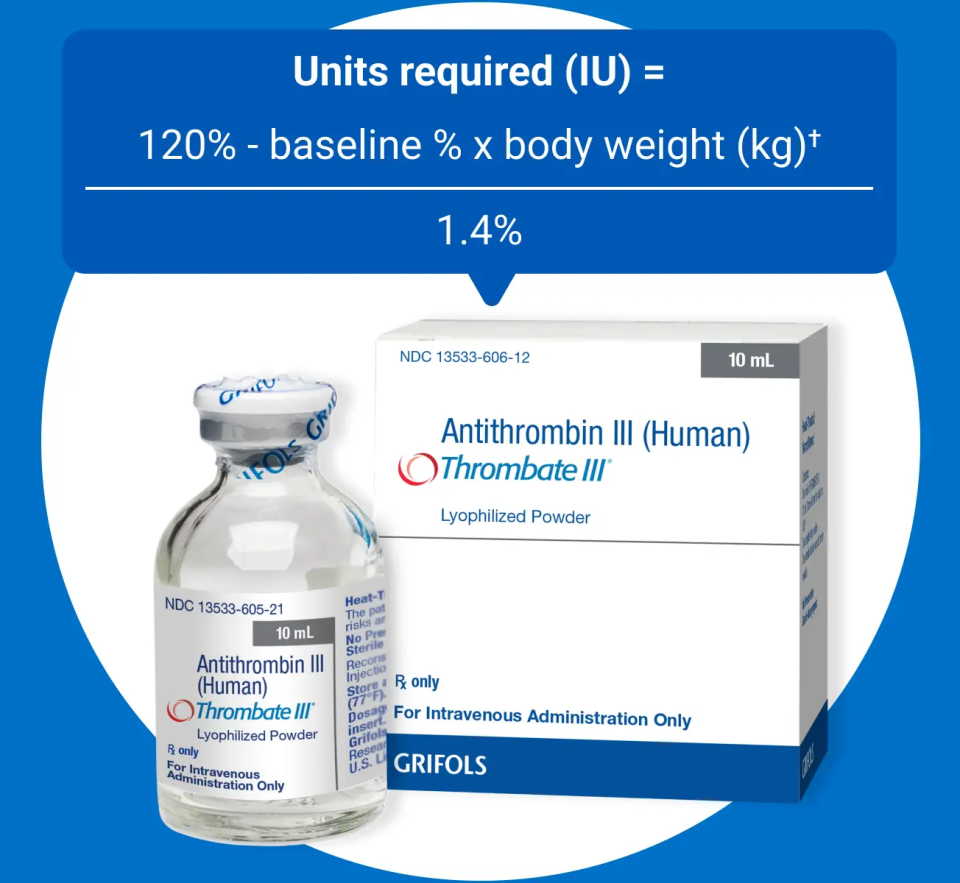
Dosing
- THROMBATE III1
-
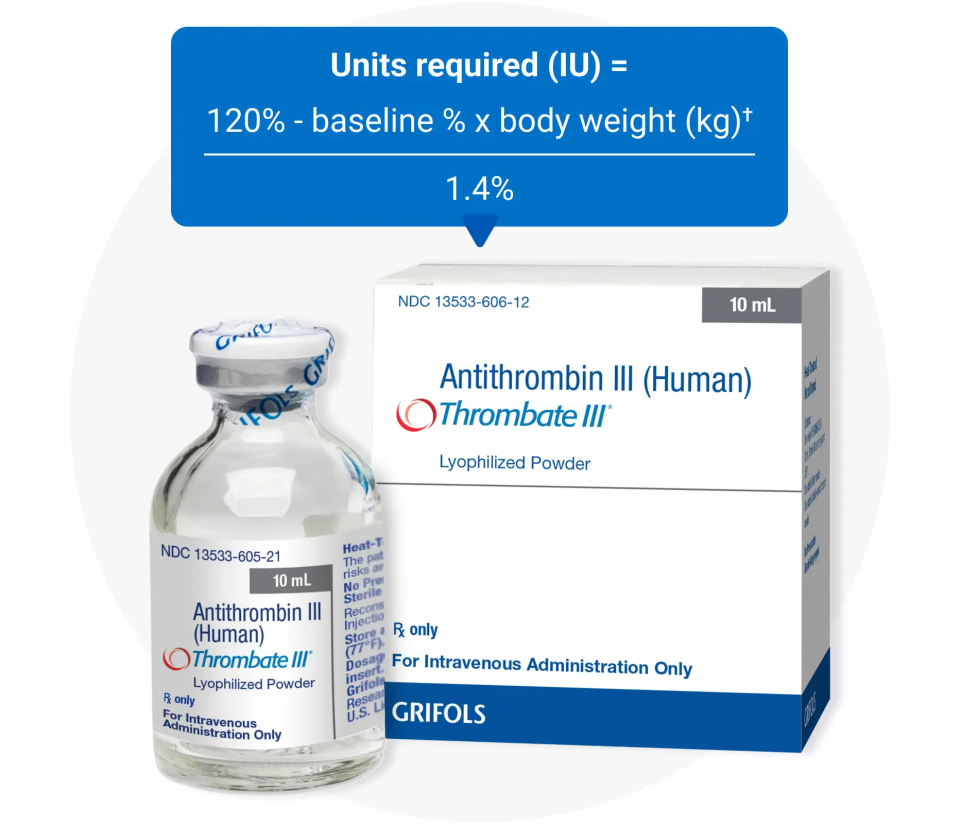
The loading dose for Thrombate III is calculated with a clear formula:
Units required (IU) = 120% - baseline % x body weight (kg)/1.4%
- FFP13
-
The volume of FFP transfused depends on various factors, including the clinical situation and patient weight, and may be guided by laboratory assays of coagulation function
| THROMBATE III1 | FFP13 | |
|---|---|---|
|
Indication |
Indicated in both pediatric and adult patients with hereditary antithrombin deficiency (hATd) for treatment and prevention of thromboembolism and for prevention of perioperative and peripartum thromboembolism |
Indicated in the management of patients with selected coagulation factor deficiencies, congenital or acquired, for which no specific coagulation concentrates are available |
|
How supplied/volume |
Single-dose 10-mL vial (500-IU potency) |
Supplied in 200- to 250-mL bags (on average) |
|
Concentration |
50 IU/mL AT concentration (after reconstitution with 10 mL sterile water for injection) |
~1 IU/mL AT concentration |
|
Use |
Intravenous bolus infusion, regardless of ABO status. Can be readily available at the point of care |
Needs to be thawed prior to use. Intravenous infusion. Plasma must be ABO compatible with the recipient's red blood cells |
|
Content |
THROMBATE III provides predictable amounts of AT |
Contains AT plus other plasma components in varying levels |
|
Half-life |
The half-life of THROMBATE III is similar to endogenous AT1,14 |
The components of FFP have varying half-lives |
|
Storage |
THROMBATE III can be stored at room temperature (up to 77°F) for up to 36 months. Do not freeze |
FFP should be stored at −18°C (0°F) or colder. Infuse immediately after thawing or store at 1-6°C (34-43°F) |
|
Process |
THROMBATE III is produced from human plasma— it is fractionated and purified to yield concentrated antithrombin |
Centrifuged, separated, and frozen solid at −18°C (0°F) within 8 hours of collection |
|
Dosing |
The loading dose for Thrombate III is calculated with a clear formula: |
The volume of FFP transfused depends on various factors, including the clinical situation and patient weight, and may be guided by laboratory assays of coagulation function |
- Predictable dosing that directly replaces the missing antithrombin (AT)
- Concentrated amounts of AT to minimize additional volume load
- Rapid preparation at the point of care—no thawing needed
- Convenient vial storage for up to 36 months at room temperature
The half-life of AT has been reported to be shortened following surgery, hemorrhage or acute thrombosis, and during intravenous heparin (or low molecular weight heparin) administration. In such conditions, monitor plasma AT levels more frequently, and administer THROMBATE III as necessary.
THROMBATE III provides a direct approach to managing hereditary AT deficiency in high-risk situations1
- The loading dose volume for THROMBATE III is calculated with a clear formula so each dose is precise and accurate
- Healthcare professionals have been successfully treating hereditary AT deficiency with THROMBATE III for more than 25 years
Learn more about THROMBATE III
Have a question about THROMBATE III?
Get in touch with a representative today
Important Safety Information
THROMBATE III® (antithrombin III [human]) is indicated in patients with hereditary antithrombin deficiency for treatment and prevention of thromboembolism and for prevention of perioperative and peripartum thromboembolism.
Hypersensitivity reactions may occur. Should evidence of an acute hypersensitivity reaction be observed, promptly interrupt the infusion and begin appropriate treatment.
Because THROMBATE III is made from human blood, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. There is also the possibility that unknown infectious agents may be present in the product.
Perform coagulation tests to avoid excessive or insufficient anticoagulation and monitor for bleeding or thrombosis. Measure functional plasma AT levels with amidolytic or clotting assays; do not use immunoassays.
In clinical studies, the most common adverse reactions (≥ 5% of subjects) were dizziness, chest discomfort, nausea, dysgeusia, and pain (cramps).
The anticoagulant effect of heparin is enhanced by concurrent treatment with THROMBATE III in patients with hereditary AT deficiency. Thus, in order to avoid bleeding, the dosage of heparin (or low molecular weight heparin) may need to be reduced during treatment with THROMBATE III.
Please see full Prescribing Information for THROMBATE III.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit http://www.fda.gov/medwatch, or call 1-800-FDA-1088.
References
- THROMBATE III [Prescribing Information]. Research Triangle Park, NC: Grifols Therapeutics LLC 2025.
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis, and treatment options. Drugs. 2007;67(10):1429-1440.
- Li W, Johnson DJD, Esmon CT, Huntington JA. Structure of the antithrombin–thrombin–heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004;11(9):857-862.
- James AH, Konkle BA, Bauer KA. Prevention and treatment of venous thromboembolism in pregnancy and patients with hereditary antithrombin deficiency. Int J Womens Health. 2013;5:233-241.
- Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21(3):131-142.
- Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24): 2482-2494.
- Kottke-Marchant K, Duncan A. Antithrombin deficiency: issues in laboratory diagnosis. Arch Pathol Lab Med. 2002;126(11):1326-1336.
- Mitton BA, Steineck A. Antithrombin deficiency. eMedicine from WebMD. http://emedicine.medscape.com/article/198573-overview. Updated July 22, 2022. Accessed November 20, 2025.
- Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229-1239.
- Pabinger I, Schneider B. Thrombotic risk in hereditary antithrombin III protein C, or protein S deficiency. Arteroscler Thromb Vasc Biol. 1996;16(6):742-748.
- Ranucci M. Antithrombin III: key factor in extracorporeal circulation. Minerva Anestesiol. 2002;68(5):454-457.
- Foy P, Moll S. Thrombophilia: 2009 update. Curr Treat Options Cardiovasc Med. 2009;11(2):114-128.
- AABB, American Red Cross, America’s Blood Centers, Armed Services Blood Program. Circular of information for the use of human blood and blood components. https://www.aabb.org/docs/default-source/default-document-library/resources/circular-of-information-watermark.pdf?sfvrsn=7f5d28ab_5 June 2024. Accessed November 20, 2025.